How 15-year-old Lucy is helping to shape Australia’s COVID-19 vaccine program
Lucy Willis with Professor Katie Flanagan, who is leading Tasmania’s participation in the PICOBOO trial.
September 7, 2022
Tasmanian patients are needed for a first-of-its-kind medical research project evaluating the best COVID-19 booster vaccine strategies in Australia..
The Platform Trial In COVID-19 Vaccine BOOsting (PICOBOO) study is a multi-centre trial initiated by the Telethon Kids Institute in Western Australia, with patients being recruited through participating sites in WA, SA and Tasmania.
The study is designed to evaluate different COVID-19 vaccine booster strategies.
Participation will help researchers determine the most effective, long-term strategies for COVID-19 booster vaccinations nationwide.
It is funded by a grant from the Medical Research Future Fund (MRFF).
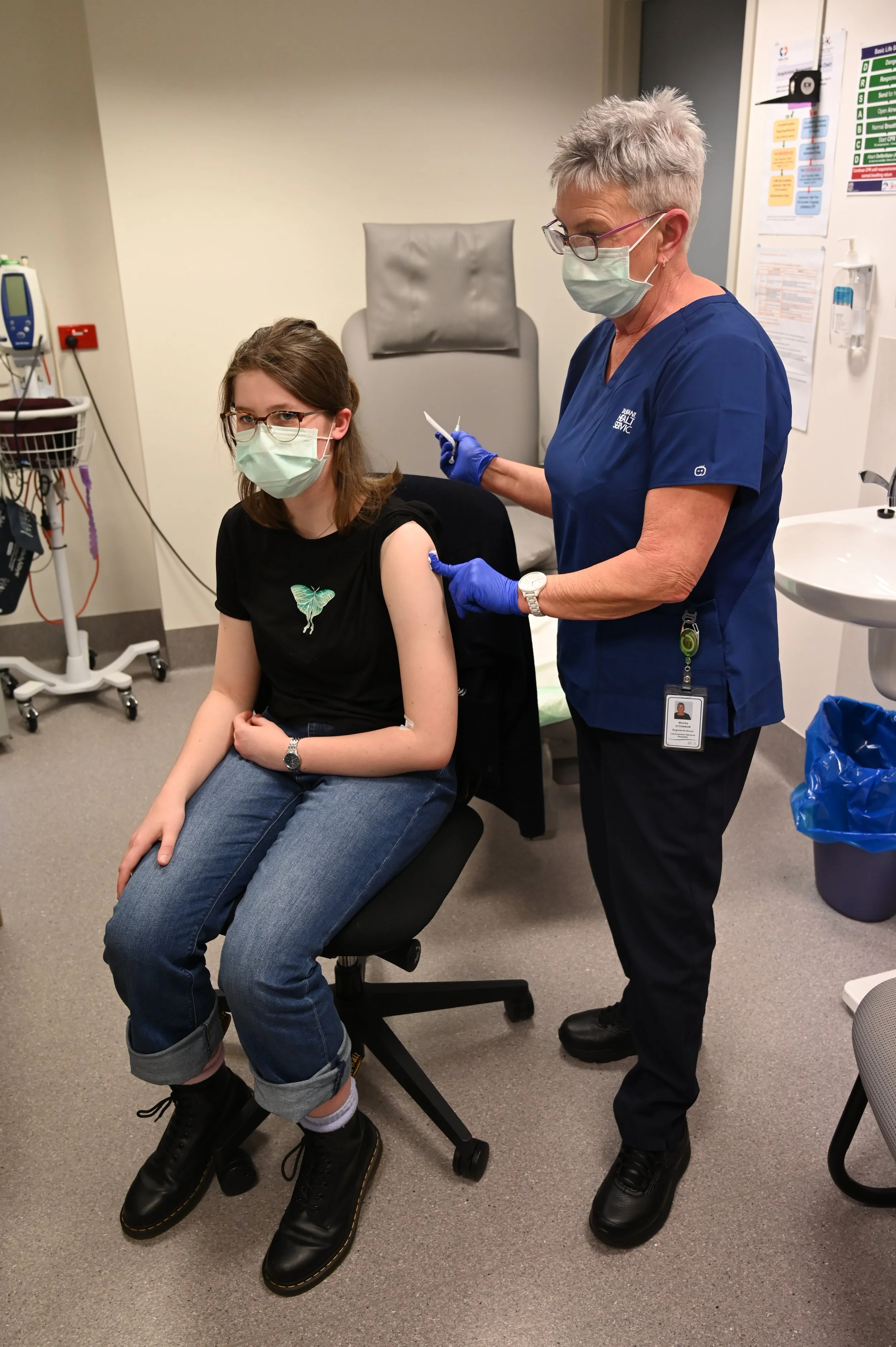
Lucy receives her booster from LGH research nurse Monika O’Connor.
Infectious Diseases Specialist Professor Katie Flanagan is leading Tasmania’s participation in the trial, out of the Tasmanian Vaccine Trial Centre at the Launceston General Hospital.
Last week 15-year-old St Aloysius Catholic College student Lucy Willis became the first Tasmanian patient recruited to the trial, driving up to Launceston from Hobart with her mother Leah.
Currently, the Australian Technical Advisory Group on Immunisation does not recommend that adolescents aged 12-15 years receive a booster dose of the COVID-19 vaccine.
However, the PICOBOO trial aims to determine the effect of COVID-19 vaccination in that age group in order to inform future policy in Australia.
PICOBOO is accepting patients aged 12 to 70 who have received two doses of Pfizer, or those aged 50 and over who have received two doses of AstraZeneca.
When she heard about the trial Lucy said she jumped at the chance to be able to get a COVID-19 booster shot and help protect others.
“It’s nice to know you are helping people out,” she said.
“I think it’s a really good thing to do, especially as it benefits you and a lot of other people as well.”
Professor Flanagan said they were trying to better understand the effects of booster vaccines, in the Australian context.
“Lots of places around the world had waves of COVID-19 before vaccines were available. But many Australians were vaccinated before they had contracted COVID-19,” Professor Flanagan explained.
“So, it’s a different setting and we need to understand the best strategies going forward in Australia.”
Professor Flanagan said researchers would examine the quality of the immune response and how long that immune response lasts, with the trial evaluating Pfizer, Moderna and Novavax COVID-19 vaccines as first or second dose booster vaccinations.
For more information contact the research team at the Tasmanian Vaccine Trial Centre based at the Clifford Craig Foundation, Launceston General Hospital by phone: (03) 6777 6010, mobile: 0474 516 022 or email: research@cliffordcraig.org.au.
Listen to Professor Flanagan speaking about the trial on Tasmania Talks here.